Timisoara_Med 2023, 2023(1), 4; doi:10.35995/20230104
Case Report
Trastuzumab-Induced Cardiac Dysfunction. A Case Report
1
Department of Cardiology, Pius Brinzeu Clinical Emergency County Hospital Timisoara, 300736 Timisoara, Romania; dr_apostol@yahoo.co.uk (A.A.); oana.ciolpan@umft.ro (O.S.); carina.bogdan@umft.ro (C.B.); ivan.viviana@umft.ro (V.I.)
2
Department of Internal Medicine II, Division of Cardiology, “Victor Babes” University of Medicine and Pharmacy Timisoara, 300041 Timisoara, Romania
*
Correspondence: lari94_ia@yahoo.com
How to cite: Dăniluc, L.; Apostol, A.; Sandu, O.; Bogdan, C.; Ivan, V. Trastuzumab-Induced Cardiac Dysfunction. A Case Report. Timisoara Med. 2023, 2023(1), 4; doi:10.35995/20230104.
Received: 13 May 2023 / Accepted: 27 June 2023 / Published: 4 July 2023
Abstract
:Oncological pathology and the management of patient with cancer are real challenges in cardiology practice. Unpredictable situations triggered by this pathology, as well as adverse reactions of oncological therapy on the cardiovascular system, are increasing. A 71-year-old female patient who was known with HER2-positive breast cancer, with sectorial mastectomy and treated with chemo-radio-immunotherapy in 2018, then diagnosed with liver metastases and ulcerated gastric adenocarcinoma in May 2022, was referred to the Cardiology Clinic for chest pain and dyspnea. On the ECG, ST-segment depression was seen in the infero-lateral territory. Positive myocardial necrosis enzymes were documented, and also moderate microcytic hypochromic anemia. The current clinical, electrical, and biological picture was suggestive for acute coronary syndrome. It should be mentioned that the patient has been on monoclonal antibody therapy with Trastuzumab, recently discontinued after echocardiographic evaluation where decreased systolic performance was noted. Considering all the particular aspects of this case, as cardiotoxicity of oncological therapy, acute coronary event and anemic syndrome, multidisciplinary management is required, with adjustment of cardiovascular therapy and cancer treatment.
Keywords:
trastuzumab; cardiotoxicity; cardiac dysfunctionIntroduction
Oncological pathology is very complex and new therapies are constantly developing. Fast-moving pace of new oncology treatment developments goes against a background of dynamic CV toxicity [1]. This clinical case shows the risks of cardiovascular damage in cancer patients due to the procoagulant and pro-inflammatory status of the pathology itself, and also due to the cancer treatment. Acute coronary syndrome in a patient with HER2-positive metastatic breast cancer, cardiotoxicity of oncological treatment and anemia are aspects that complicate the therapeutic approach and require multidisciplinary management.
Case Presentation
A 71-year-old female patient with multiple cardiovascular risk factors was referred to the Cardiology Clinic with chest pain, dyspnea and altered general condition. The patient was diagnosed in 2018 with right breast neoplasm with the presence of HER-2 mutation, and underwent sectorial mastectomy followed by chemo-radio-immunotherapy. In May 2022 she was diagnosed with liver metastases and gastric cancer (ulcerated adenocarcinoma, HER-2-positive), and treated with immunotherapy using Trastuzumab and associating hormone therapy with Fulvestrant. Eight months after the initiation of the oncological therapy (in December 2022), the patient underwent a cardiological evaluation for dyspnea with orthopnea and marked fatigue, and based on the biological findings (increased NT-ProBNP values) and echocardiographic examination (decreased systolic performance, EF = 35%), was diagnosed with heart failure with reduced EF, due to Trastuzumab cardiotoxicity [1].
At the admission in the Emergency Department in February 2023, the patient had altered general condition, BP = 160/100 mm Hg, HR = 120 bpm, rhythmic, tachycardic heart sounds. Myocardial necrosis enzymes values were elevated and she had moderate microcytic hypochromic anemia. The ECG showed ST-segment depressions of approximately 2 mm in the infero-lateral territory. The echocardiography documented LV with global hypokinesia, with significant valvular disease, severe mitral regurgitation by mixed mechanism, moderate functional tricuspid regurgitation and mild pulmonary hypertension (Figure 1 and Figure 2). She was admitted to the Coronary Intensive Care Unit with the diagnosis of non-ST elevation acute coronary syndrome.
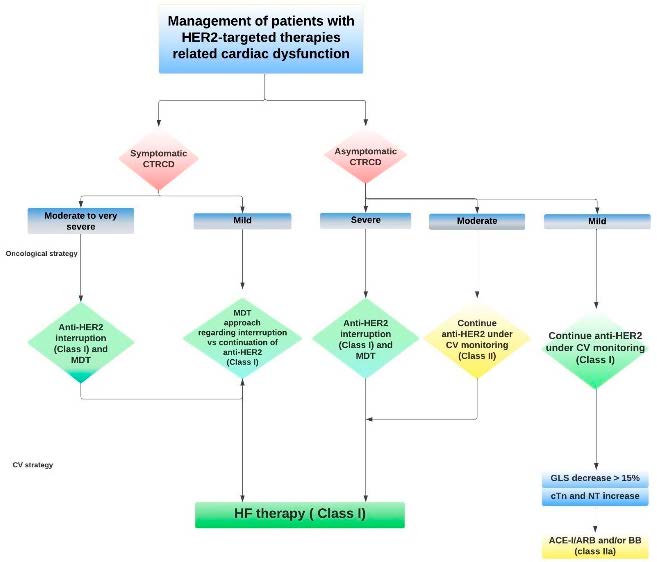
What is the therapeutic management in the case of a patient with cancer, who has signs of cardiac decompensation in the context of cardiotoxicity, but also with acute coronary syndrome in a short period of time, associated with moderate secondary anemia? Taking each particular aspect into account and based on clinical judgment, but also on sources such as 2022 ESC Guidelines, it was decided to temporize the oncological therapy (considering the cardiotoxicity effect) (Figure 3).
Anemia was corrected, and for the acute coronary event, a conservative therapy approach was chosen, with the administration of double antiplatelet therapy, kept only for one month (considering the high risk of bleeding), of a statin and a beta-blocker. The therapy for heart failure was also adjusted, and sacubitril/valsartan and dapagliflozin were introduced.
The cardiological outcome was favorable, with partial improvement of symptoms, but the choice of oncological therapy in this patient with documented cardiotoxicity following monoclonal antibody treatment is a real challenge and requires periodic cardiovascular follow-up.
Discussion
From 1990 to the present there has been a decline in cancer mortality and so an increase in the number of cancer survivors has been reported. In this context, the side effects of cancer therapy have gained importance. Cardiovascular toxicity is one of the major side effects of cancer therapy, so choosing the optimal therapy, taking into account the individualized cardiovascular risk, has a great impact on the long-term mortality and morbidity outcomes of cancer patients. Effective management of patients presenting with cardiovascular disease and diagnosed with cancer, as well as cardiovascular disease, requires the additional attention of a multidisciplinary team. Some oncology drugs are associated with a specific cardiotoxicity profile. Two classic types of cardiotoxicity are described: Type 1—irreversible cardiac injury caused by anthracyclines; Type 2—reversible cardiotoxicity caused by monoclonal antibodies [1].
Several studies have been published on this very interesting and actual topic. For example, in a study conducted on a cohort of 227 patients treated with Trastuzumab, the prevalence of cardiotoxicity was 17.6% (2.2% symptomatic, 15.4% asymptomatic); it should be mentioned that cardiotoxicity was defined as a decrease in left ventricle ejection fraction (LVEF) below 50%, as an absolute decrease of >10 points from baseline, or as any evidence of heart failure [2]. In another study investigating the effect of Trastuzumab on cardiac function in a cohort of patients with HER2-positive metastatic breast cancer, but also with reduced LVEF at baseline, it was reported that 65% of patients who received Trastuzumab despite having a compromised baseline LVEF, did not develop severe cardiotoxicity during an 18-month follow-up [3]. In the cases where severe cardiotoxicity was reported, it was at least partially reversible in more than two-thirds of cases [4,5]. The benefits and risks of trastuzumab use need to be carefully evaluated in this vulnerable population [6]. An important aspect of the ESC Guidelines on Cardio-Oncology is the reversibility of cardiac injury once trastuzumab treatment is interrupted. Therefore, as a class IC indication, in patients who develop severe heart failure, temporary discontinuation of treatment is highly recommended. In cases of asymptomatic moderate and mild heart failure, initiation of heart failure treatment and a more frequent follow-up is recommended [1].
In this complex case, the 2022 ESC Guidelines on Cardio-Oncology have been an extremely important management tool. The therapeutic management chosen in this situation was individualized, guided by the clinical experience and by the associated pathologies of the patient, but in accordance with the information studied and published in the ESC Guidelines. Considering that CV toxicity risk is a dynamic variable, an initial CV risk assessment is recommended for all cancer patients who are about to receive potentially cardiotoxic anticancer treatment [7]. This allows oncologists to consider CV risk while choosing optimal oncological treatment, educate patients about their CV risk, and individualize the follow-up strategy [1].
Conclusions
Primary prevention of cardiotoxicity of cancer therapy intends to avoid or reduce the occurrence of cardiovascular adverse events in patients without previously documented cardiovascular disease. Secondary prevention involves interventions in patients with already documented cardiovascular disease, including previous or new CTR-CVT [8]. It is necessary to establish a plan to prevent the development of cardiotoxicity and also, of an accurate surveillance plan for potential CV complications. The optimal management of CV risk factors and pre-existing cardiovascular disease is essential to facilitate cancer therapy and improve prognosis. If patients are carefully followed during cancer therapy, including cardiac biomarkers, echocardiography, GLS, it is possible to detect CV toxicity as a result of specific oncological therapies [1].
Author Contributions
Conceptualization, A.A. and V.I.; Methodology, L.D. and V.I.; Formal Analysis, L.D., O.S. and C.B.; Investigation, L.D., O.S. and C.B.; Data Curation, A.A.; Writing—Original Draft Preparation, L.D.; Writing—Review & Editing, A.A. and V.I.; Visualization, L.D., A.A. and V.I.; Supervision, V.I.; Project Administration, L.D. and V.I.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Torelli, F.; Ghiselli, L.; Rossi, A.; Trevisani, L.; Vinco, G.; Truong, S.; Benfari, G.; LARussa, F.; Golia, G.; et al. Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: results from a single-center retrospective study. Int. J Cancer 2022, 151, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Bouwer, N.I.; Steenbruggen, T.G.; Rier, H.N.; Kitzen, J.J.E.M.; Smorenburg, C.H.; van Bekkum, M.L.; de Jong, P.C.; Drooger, J.C.; Holterhues, C.; Kofflard, M.J.M.; et al. The effect of trastuzumab on cardiac function in patients with HER2-positive metastatic breast cancer and reduced baseline left ventricular ejection fraction. Breast Cancer Res. Treat. 2021, 186, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, M.; Al-Habbaa, A.; Abdelshafy, M.; Davey, M.G.; Elkoumy, A.; Ganly, S.; Elzomor, H.; Cawley, C.; Sharif, F.; Crowley, J.; et al. Genetic and RNA-related molecular markers of trastuzumab-chemotherapy-associated cardiotoxicity in HER2 positive breast cancer: a systematic review. BMC Cancer 2022, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Xiong, W.; Wang, S.; Li, Y.; Hou, C.; Li, C.; Li, G. The Research Progress of Trastuzumab-Induced Cardiotoxicity in HER-2-Positive Breast Cancer Treatment. Front. Cardiovasc. Med. 2022, 8, 821663. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, N.; Rosenthal, A.; Dabas, N.; Kropotova, Y.; Lippman, M.; Bishopric, N.H. Trastuzumab-induced cardiotoxicity: A review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies. Breast Cancer Res. Treat. 2021, 188, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Chianca, M.; L'Abbate, S.; Fabiani, I.; Aimo, A.; Emdin, M.; Passino, C.; Fedele, A.; Cipolla, C.M.; Cardinale, D.M. Clinical management of drug-induced cardiotoxicity in patients with HER-2+ breast cancer: current recommendations and future outlook. Expert Opin. Drug Metab. Toxicol. 2023, 19, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xu, R.; Zhou, B.; Lin, C.; Guo, Y.; Xu, H.; Guo, X. Clinical Manifestations, Monitoring, and Prognosis: A Review of Cardiotoxicity After Antitumor Strategy. Front. Cardiovasc. Med. 2022, 9, 912329. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Ultrasonographic parasternal long axis view.
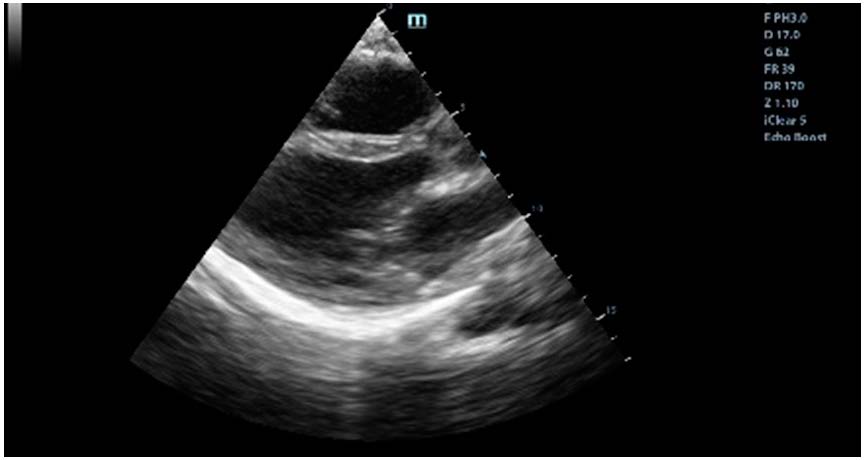
Figure 2.
Ultrasonographic aspect of severe mitral regurgitation.
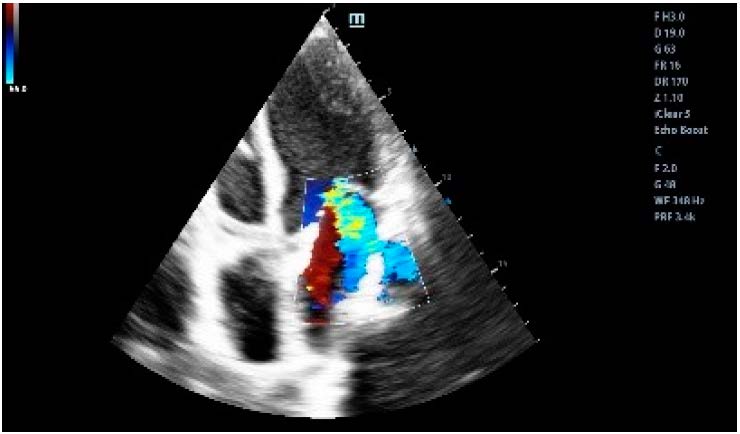
Figure 3.
Management of targeted HER-2 therapy related cardiac dysfunction (adapted from [1]).
Figure 3.
Management of targeted HER-2 therapy related cardiac dysfunction (adapted from [1]).
© 2023 Copyright by the authors. Licensed as an open access article using a CC BY 4.0 license.
