Timisoara_Med 2021, 2020(2), 7; doi:10.35995/tmj20200207
Review
Ring-Modified Triterpene Derivatives as Potential Pharmacological Active Compounds
Faculty of Pharmacy, “Victor Babes” University of Medicine and Pharmacy, 2 E. Murgu Sq., 300041 Timisoara, Romania; ulicialexandra@yahoo.com (A.U.); andreea.milan@yahoo.com (A.M.); roxana.ghiulai@umft.ro (R.G.); babuta.roxana@umft.ro (R.R.); codrutasoica@umft.ro (C.Ș.)
*
Correspondence: marius.mioc@umft.ro; Tel.: +40-756-236-715
†
Authors with equal contribution, sharing the position of first author.
How to cite: Ulici, A.; Milan, A.; Mioc, M.; Ghiulai, R.; Racoviceanu, R.; Șoica, C. Ring Modified Triterpene Derivatives as Potential Pharmacological Active Compounds. Timisoara Med. 2020, 2020(2), 7; doi:10.35995/tmj20200207.
Received: 11 December 2020 / Accepted: 4 February 2021 / Published: 18 February 2021
Abstract
:Pentacyclic triterpenes represent an important class of intensively studied substances in the past two decades due to their wide spectrum of pharmacological effects. Even though this class is being thoroughly researched for the development of new drugs, to date, no representative has qualified to become a suitable candidate for various therapies. Although a large part of past and ongoing studies focuses on triterpene chemical derivatization or formulation to increase its bioavailability, other researchers are focused on obtaining semisynthetic derivatives not necessarily with increased hydrophilicity but with a superior biological effect compared to their parent compound. The current review aims to report the biological assessment of several ring-modified pentacyclic triterpene derivative series.
Keywords:
pentacyclic triterpenes; ring derivatization; synthetic derivatives; biological activityIntroduction
In developing countries, traditional medicine based on natural remedies remains the principal treatment for more than 70% of the population, the main reason being the impossibility to access modern drugs [1]. Currently, natural remedies are becoming increasingly used in developed countries as well, due to the increased pollution and rising incidence of chronic stress, which have induced an increased interest in the general health of the population [2].
Nowadays, natural compounds play an important role in the discovery and development of new therapeutic agents, acting as starting points for the synthesis of various new derivatives through different chemical reactions. Frequently, the newly obtained semisynthetic derivatives show improved pharmacological proprieties [3].
Pentacyclic triterpenes are widely distributed in nature and can be found in numerous medicinal plants, fruits, cereals and vegetable oils. The healthful effects of the Mediterranean diet are also associated with the triterpene content of the fruits and vegetables consumed [4].
Pentacyclic triterpenes are secondary metabolites composed of isoprene units synthesized through the cyclization of epoxidized squalene in the cytosol [5]. From a chemical perspective, the group of pentacyclic triterpenes is composed of three main subclasses, based on the main scaffold: lupane, oleanane and ursane derivatives [4].
Pentacyclic triterpenes are a class of natural compounds that have received considerable attention as a result of their broad spectrum of biological effects, including antitumor, anti-inflammatory, cardio-protective, vasodilatory and antioxidant activities [4,6].
A well-known problem of pentacyclic triterpenes is their low bioavailability caused mainly by their high lipophilicity and water insolubility, which reduce gastrointestinal absorption. Various strategies have been developed in order to overcome this obstacle, including the chemical derivatization of the compounds as well as liposomes and nanoparticles formulation [4].
The present review aims to present a short overview of recent studies that have been conducted to determine the therapeutic potential of newly obtained ring-modified triterpene derivatives.
Lupane Ring-Modified Compounds
The lupane group is a subdivision of the pentacyclic triterpenes main group, which includes lupeol, betulin and betulinic acid [4]. Lupane-type triterpenoids are widely spread throughout the plant kingdom and possess a wide panel of biological activities, including antibacterial, antiviral and anticancer properties. In recent years, lupane-type triterpenoids have been subjected to numerous derivatizations aimed to improve their bioavailability and enhance their therapeutic effects [7]. For example, to increase the cytotoxicity of betulinic acid, the majority of studies focused on the derivatization of the A-ring, C-3 hydroxyl, C-20 alkene and C-28 carboxylic acid moieties [8].
The in vitro activity against Mycobacterium tuberculosis was evaluated for a series of A-ring azepane and azepinone derivatives of pentacyclic triterpenes. The newly synthesized A-azepano-28-hydroxy derivatives did not induce an increase in the antimycobacterial activity (Figure 1A). The A-azepano-28-cinnamoyloxybetulin proved to be a lead compound with strong activity against the well-known H37Rv strain, with a minimum bactericidal concentration value of 4 µM as well as a series of resistant strains, including isoniazid-resistant strain (INH-R), ofloxacin-resistant strain (OFX-R) and rifampicin-resistant strain (RIF-R), with minimum inhibitory concentration values of 4, 1 and 1 µM, respectively (Figure 1B) [9].
Six A-seco-amino derivatives were included in a study that conducted the in vitro evaluation of the anti-influenza type-A activity of a series of lupane-type pentacyclic triterpenes (Figure 1C). The compounds proved to be more active against the H1N1 strain than the initial acanthochlamic acid; however, the activity against the H3N2 strain was nearly nonexistent [10].
Haavikko et al. synthesized and tested against Leishmania donovani a series of A-ring fused heterocycles of betulinic acid. After the primary evaluation, the A-ring fused pyrazine derivative (compound 5) and the 4-Aza-3-oxohomobetulinic acid (compound 8) showed significant parasite growth inhibition, even in low concentrations (5 µM), with IC50 values of 13.2 and 4.3 µM, respectively (Figure 2). Between the two compounds, compound 8 revealed a higher selectivity index and activity against infected macrophages and axenic amastigotes [11].
In an extensive study conducted by Kazakova et al., a series of C28-amino lupane derivatives were synthesized in order to evaluate their in vitro cytotoxicity against a panel of 60 cancer cell lines. A-azepano-28-amino-betulin derivatives showed notable activities against all cell lines, with IC50 values ranging between 1.16 and 2.27 µM (Figure 2). The highest measured activities were recorded towards leukemia, breast cancer, colon cancer and nonsmall cell lung cancer cell lines. The replacement of the hydroxyl group at C28 of the A-azepano-betulin with an amino group did not significantly improve the cytotoxicity of the obtained derivatives [7].
A recent study described the development of new cholinesterase inhibitors based on A-ring azepano-triterpenoids and 3,4-seco derivatives. The synthesized compounds revealed a mixed-type inhibitory effect on both acetylcholinesterase and butyrylcholinesterase. Between the evaluated compounds, a C28-amide derivative of azepanobetulin (compound 8) showed a promising cholinesterase inhibitory effect, being approximately ten times more active than the reference compound, galantamine hydrobromide (Figure 2) [12].
It was reported that lupane-type triterpenoids possess antidiabetic proprieties through various mechanisms including the inhibition of α-glucosidase [13,14]. In a study that highlighted the key role of the amide side chain in C28 for the α-glucosidase inhibitory effect, an imidazole amide with A-lactam cycle (compound 21) proved to be 30 times more active than the standard acarbose, with an IC50 value of 6.19 µM. In the same study, complete loss of activity was reported for the A-azepanone-17-cyano-lup-20(29)-ene derivative (compound 17) [15].
Maldonado et al. isolated lippiolide and lippiolic acid, two new lupane-type triterpenoids found in Lippia mexicana, and evaluated their anti-inflammatory activity on the TPA-induced mouse ear edema model. Lippiolide, containing a δ-lactone at ring E, showed significant edema reduction (74.9%); however, the IC50 value (0.73 µM) was three times higher than the value recorded for the standard indomethacin (IC50 0.24 µM) (Figure 2). The edema reduction induced by lippiolic acid was significant (74.9%), but the IC50 value could not be determined due to its low solubility at concentrations over 1 µM [16].
Oleanane Ring-Modified Compounds
Oleanolic acid is among the most studied pentacyclic triterpenes, which can be found in more than 1600 species of plants worldwide and exhibits a wide range of pharmacological proprieties, such as antiviral, anticancer and antibacterial [3]. Due to its polycyclic structure, which has allowed various chemical transformations, the pharmacological activity of the main compound has been improved by chemically modifying the oleanane ring, as reported by various in vivo and in vitro studies. Oleanolic acid possesses three active sites, C3-hydroxyl, C12-C13 double bond in ring-C and C28-carboxylic acid, which can be chemically modulated for an optimized pharmacologic effect [17].
An improved antiviral activity was noticed for the O-acyloxyamino derivates of oleanane; these derivates were tested comparatively with the main compound, oleanolic acid, in terms of their activity as inhibitors of HIV-1 dimerization protease. The acyl function has proved to be responsible for a stronger inhibition of the enzyme; furthermore, the acylated oximes of oleanolic acid also revealed higher antifungal activity than the parent compound (Figure 3) [3].
In another study conducted by Wei Cui et al., the antimuscle atrophy effect of oleanolic acid was compared to one of its derivatives, HA-19, through in vitro and in vivo experiments (Figure 3). Firstly, in vitro models were used to assess myoblast differentiation and myotube atrophy in order to establish the inhibitory capacity of oleanolic acid and its derivatives on muscle atrophy. The most promising derivate was HA-19, which was able to up-regulate the protein synthesis pathway and also prevent protein degradation by down-regulating the negative growth factors. The antimuscle atrophy effect was confirmed by an in vivo disuse-induced muscle atrophy model [18].
The antiosteoporotic propriety of oleanolic acid and its derivatives was assessed by Jun-Feng Li et al.; a preliminary analysis of oleanolic acid’s derivates demonstrated that hydrophobic groups, such as methyl ester, may act as a promoter of the antiosteoporotic activity, while the substitution of the phenyl ring with an indole moiety could also enhance the biological effect. The length of the alkyl chain in amino acid derivates was also crucial; short chains, such as alanine and glycine, trigger an intensified activity, while long chains might be detrimental to the pharmacological activity [19]. In order to evaluate the antimicrobial activity, oleanolic acid was derivatized at the amide bond with diethylenetriamine; the new compound showed promising activity against both Gram-negative and Gram-positive bacteria. Although the derivative cannot be put in use yet, in vivo studies aiming to investigate its cytotoxicity are currently underway [20].
The cytoprotective and anti-inflammatory activity of oleanolic acid was assessed comparatively to its derivatives in a study conducted by Kuzniak et al. The higher biological effect exhibited by the semisynthetic derivatives might be correlated to oleanolic acid’s low bioavailability caused by its hydrophobic character. In order to increase its bioavailability, oxime derivatives were conjugated with succinic acid at the C-3 position, each derivative differing from the other through various substituents in the C-17 position. Each derivative was tested for its effect on the signaling pathways involved in inflammation; the results of the study indicated that morpholide derivative acted as the most efficient down regulator of the tested pathways [21].
Another study focused on the pro-apoptotic propriety of oleanolic acid and its derivates on breast cancer cells. The antitumor activity of the oleanolic acid was confirmed by its inhibitory effect on tumor cell promotion and angiogenesis, although its low efficiency caused by its physicochemical properties raised the need to design semisynthetic derivates that could significantly increase its anticancer activity. The oleanolic acid was modified at positions C3, C11 and C28 of the main scaffold, by inserting hydroxyimin and keto groups; the introduction of the C3-hydroxyimin group induced not only a higher inhibition of tumor cell proliferation but also the ability to cause cell death by apoptosis or autophagy. An MTT assay was performed on the oleanane derivates in order to select the one which possess the strongest cytotoxic effect against cancer cells; it revealed that the highest pro-apoptotic effect was exerted by HIMOXOL (Methyl 3-hydroxyimino-11-oxoolean-12-en-28-oate) (Figure 3) [22].
In order to solve the issue of chemotherapy failure in hematological malignancies caused by multidrug resistance, a study describing the development of a set of oleanolic acid derivatives was conducted. Oleanolic acid was modified at C3, C11, C12 and C28 positions; the propriety of multidrug resistance modulator was tested on human lymphoblastic leukemia cell lines. The most promising compound was DIOXOL (methyl 3,11-dioxoolean-12-en-28-olate), which showed a significantly higher effect than the parent compound (Figure 3) [23].
The cytotoxic evaluation of oleanolic acid and its derivatives has been again performed by MTT assay on human cancer lines in another study conducted by Pattnaik et al. The most efficient semisynthetic derivative, which induced a mediated apoptosis and withheld the cell cycle in G0/G1, was a compound containing a conjugated double bond system in ring C; its cytotoxic activity was significantly increased compared to the parent compound [17].
Another study focused on oleanolic acid semisynthetic derivatives that contain an α,β-unsaturated carbonyl moiety; their anticancer activity was assessed in vitro by means of MTT assay. Most derivatives showed an improved inhibitory cell growth activity compared to the parent compound; by modifying the carboxylic moiety and introducing an amidic bond, a semisynthetic derivative that can significantly induce cell apoptosis and arrest the cell in S phase was obtained [24].
Oleanolic and ursolic acids were derivatized in order to highlight their potential for inhibiting cholinesterases in the attempt to improve life quality for people who suffer from neurodegenerative diseases. Whereas ursolic acid only showed inhibitory activity against acetylcholinesterase, its hydroxy-propynyl derivative exhibited promising activity against both acetylcholinesterase and butyrylcholinesterase [25].
Other Types of Modified Compounds
Glycyrrhetinic acid is another compound that belongs to the group of triterpenes which also exhibits apoptotic and growth inhibitory properties in cancer cells. The antiproliferative activity of its derivatives exerted through apoptosis induction showed a significant improvement compared to the parent compound [3].
Ursolic acid also revealed antiproliferative activity against cancer cells; due to its low bioavailability, the need to synthesize a highly active functional derivative became crucial. The main chemical functionalizations were performed at the carboxyl moiety in C28 position and on the unsaturated C-ring in the C12-C13 position; all semisynthetic derivatives revealed stronger antiproliferative activity in several cancer cell lines compared to the parent compound [26].
Boswellic acid is a pentacyclic triterpene with anticancer activity; the chemical derivatization of its skeleton leads to intensified biological effects. Some synthesized compounds were obtained through the esterification of the A-ring lactones or lactams, revealing a highly improved cytotoxicity compared to the main compound [27].
Conclusions
The current need for developing natural compounds able to offer high tolerability for patients suffering from different types of cancer, HIV, bacterial or viral infections has hit a high peak in the last decade. Triterpenes are a chemical group of compounds that possess a wide range of pharmacological proprieties, not only anticancer and antibacterial but also hepatoprotective, antioxidant, anti-inflammatory, analgesic, antihyperlipidemic and antinociceptive. Chemical transformations of these natural compounds are compulsory in order to overcome their low bioavailability due to their high hydrophobicity. The resulting semisynthetic compounds show stronger pharmacological activity compared to the parent compound. The current review covers the applications of several derivatization methods to the most frequently used triterpenes in the pharmaceutical and biomedical fields in order to augment their pharmacological properties.
Author Contributions
Conceptualization, M.M. and C.Ș.; Methodology, A.U. and A.M.; Validation, C.Ș., M.M. and R.G.; Formal Analysis, R.R.; Writing—Original Draft Preparation, A.U. and A.M.; Writing—Review and Editing, A.U., A.M. and M.M., C.Ș.; Supervision, M.M. and C.Ș.; Funding Acquisition, C.Ș.
Funding
This work was supported by an internal grant from the Victor Babeș University of Medicine and Pharmacy, Timișoara, Grant 1EXP/1233/30.01.2020 LUPSKINPATH, Project Manager: Codruta Soica
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majolo, F.; Delwing, L.K.D.O.B.; Marmitt, D.J.; Bustamante-Filho, I.C.; Goettert, M.I. Medicinal plants and bioactive natural compounds for cancer treatment: Important advances for drug discovery. Phytochem. Lett. 2019, 31, 196–207. [Google Scholar] [CrossRef]
- Džamić, A.M.; Matejić, J.S. Aromatic Plants from Western Balkans: A Potential Source of Bioactive Natural Compounds. In Active Ingredients from Aromatic and Medicinal Plants; El-Shemy, H., Ed.; IntechOpen: Rijeka, Croatia, 2019; pp. 13–15. [Google Scholar]
- Bednarczyk-Cwynar, B.; Zaprutko, L. Recent advances in synthesis and biological activity of triterpenic acylated oximes. Phytochem. Rev. 2015, 14, 203–231. [Google Scholar] [CrossRef] [PubMed]
- Furtado, N.A.J.C.; Pirson, L.; Edelberg, H.; Miranda, L.M.; Loira-Pastoriza, C.; Préat, V.; Larondelle, Y.; Andre, C.M. Pentacyclic Triterpene Bioavailability: An Overview of In Vitro and In Vivo Studies. Molecules 2017, 22, 400. [Google Scholar] [CrossRef] [PubMed]
- Gill, B.S.; Kumar, S. Navgeet Triterpenes in cancer: significance and their influence. Mol. Biol. Rep. 2016, 43, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C. Inhibitory effects and actions of pentacyclic triterpenes upon glycation. Biomedicine 2015, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Lopatina, T.V.; Baikova, I.P.; Zileeva, Z.R.; Vakhitova, Y.V.; Suponitsky, K.Y. Synthesis, evaluation of cytotoxicity, and antimicrobial activity of A-azepano- and A-seco-3-amino-C28-aminolupanes. Med. Chem. Res. 2020, 29, 1507–1519. [Google Scholar] [CrossRef]
- Kumar, P.; Bhadauria, A.S.; Singh, A.K.; Saha, S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018, 209, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, N.I.; Kazakova, O.B.; Lopatina, T.V.; Smirnova, I.E.; Giniyatullina, G.V.; Baikova, I.P.; Kataev, V.E. Synthesis and antimycobacterial activity of triterpenic A-ring azepanes. Eur. J. Med. Chem. 2018, 143, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.E.; Kazakova, O.B. Structure—Anti-influenza Type a Activity Relationship among a Series of Nitrogen Lupane Triterpenoids. Nat. Prod. Commun. 2018, 13, 1267–1270. [Google Scholar] [CrossRef]
- Haavikko, R.; Sacerdoti-Sierra, N.; Kopelyanskiy, D.; Alakurtti, S.; Tikka, M.; Jaffe, C.L.; Nasereddin, A.; Yli-Kauhaluoma, J. Heterocycle-fused lupane triterpenoids inhibit Leishmania donovani amastigotes. MedChemComm 2014, 5, 445–451. [Google Scholar] [CrossRef]
- Kazakova, O.; Smirnova, I.; Lopatina, T.; Giniyatullina, G.; Petrova, A.; Khusnutdinova, E.; Csuk, R.; Serbian, I.; Loesche, A. Synthesis and cholinesterase inhibiting potential of A-ring azepano- and 3-amino-3,4-seco-triterpenoids. Bioorg. Chem. 2020, 101, 104001. [Google Scholar] [CrossRef]
- Ding, H.; Wu, X.; Pan, J.; Hu, X.; Gong, D.; Zhang, G. New Insights into the Inhibition Mechanism of Betulinic Acid on α-Glucosidase. J. Agric. Food Chem. 2018, 66, 7065–7075. [Google Scholar] [CrossRef]
- Yu, M.-H.; Shi, Z.-F.; Yu, B.-W.; Pi, E.-H.; Wang, H.-Y.; Hou, A.-J.; Lei, C. Triterpenoids and α-glucosidase inhibitory constituents from Salacia hainanensis. Fitoterapia 2014, 98, 143–148. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Thu, H.N.T.; Tu, A.L.T.; Thanh, T.N.; Thi, C.B.; Babkov, D.A.; Kazakova, O.B. Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors. Bioorg. Chem. 2019, 88, 102957. [Google Scholar] [CrossRef]
- Maldonado, E.; Díaz-Arumir, H.; Toscano, R.A.; Martínez, M. Lupane Triterpenes with a δ-Lactone at Ring E, from Lippia mexicana. J. Nat. Prod. 2010, 73, 1969–1972. [Google Scholar] [CrossRef]
- Pattnaik, B.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Synthesis of ring-C modified oleanolic acid derivatives and their cytotoxic evaluation. Bioorg. Chem. 2016, 68, 152–158. [Google Scholar] [CrossRef]
- Cui, W.; Liu, C.-X.; Zhang, Y.-C.; Shen, Q.; Feng, Z.-H.; Wang, J.; Lu, S.-F.; Wu, J.; Li, J.-X. A novel oleanolic acid derivative HA-19 ameliorates muscle atrophy via promoting protein synthesis and preventing protein degradation. Toxicol. Appl. Pharmacol. 2019, 378, 114625. [Google Scholar] [CrossRef]
- Li, J.-F.; Zhao, Y.; Cai, M.-M.; Li, X.-F.; Li, J.-X. Synthesis and evaluation of a novel series of heterocyclic oleanolic acid derivatives with anti-osteoclast formation activity. Eur. J. Med. Chem. 2009, 44, 2796–2806. [Google Scholar] [CrossRef]
- Kazakova, O.; Brunel, J.M.; Khusnutdinova, E.; Negrel, S.; Giniyatullina, G.V.; Lopatina, T.V.; Petrova, A.V. A-Ring-Modified Triterpenoids and Their Spermidine–Aldimines with Strong Antibacterial Activity. Molbank 2019, 2019, M1078. [Google Scholar] [CrossRef]
- Krajka-Kuźniak, V.; Bednarczyk-Cwynar, B.; Narożna, M.; Szaefer, H.; Baer-Dubowska, W. Morpholide derivative of the novel oleanolic oxime and succinic acid conjugate diminish the expression and activity of NF-κB and STATs in human hepatocellular carcinoma cells. Chem. Interact. 2019, 311, 108786. [Google Scholar] [CrossRef]
- Lisiak, N.; Paszel-Jaworska, A.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Kaczmarek, M.; Rybczynska, M. Methyl 3-hydroxyimino-11-oxoolean-12-en-28-oate (HIMOXOL), a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MDA-MB-231 breast cancer cells. Chem. Interact. 2014, 208, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Paszel, A.; Rubiś, B.; Bednarczyk-Cwynar, B.; Zaprutko, L.; Kaczmarek, M.; Hofmann, J.; Rybczyńska, M. Oleanolic acid derivative methyl 3,11-dioxoolean-12-en-28-olate targets multidrug resistance related to ABCB1. Pharmacol. Rep. 2011, 63, 1500–1517. [Google Scholar] [CrossRef]
- Wang, S.-S.; Zhang, Q.-L.; Chu, P.; Kong, L.-Q.; Li, G.-Z.; Li, Y.-Q.; Yang, L.; Zhao, W.-J.; Guo, X.-H.; Tang, Z.-Y. Synthesis and antitumor activity of α,β-unsaturated carbonyl moiety- containing oleanolic acid derivatives targeting PI3K/AKT/mTOR signaling pathway. Bioorg. Chem. 2020, 101, 104036. [Google Scholar] [CrossRef] [PubMed]
- Loesche, A.; Köwitsch, A.; Lucas, S.D.; Al-Halabi, Z.; Sippl, W.; Al-Harrasi, A.; Csuk, R. Ursolic and oleanolic acid derivatives with cholinesterase inhibiting potential. Bioorg. Chem. 2019, 85, 23–32. [Google Scholar] [CrossRef]
- Mendes, V.I.S.; Bartholomeusz, G.A.; Ayres, M.; Gandhi, V.; Salvador, J.A.R. Synthesis and cytotoxic activity of novel A-ring cleaved ursolic acid derivatives in human non-small cell lung cancer cells. Eur. J. Med. Chem. 2016, 123, 317–331. [Google Scholar] [CrossRef]
- Li, T.; Fan, P.; Ye, Y.; Luo, Q.; Lou, H.-X. Ring A-modified Derivatives from the Natural Triterpene 3-O-acetyl-11-keto-β-Boswellic Acid and their Cytotoxic Activity. Anti-Cancer Agents Med. Chem. 2017, 17, 1153–1167. [Google Scholar] [CrossRef]
Figure 1.
Chemical structures of various active lupane-type derivatives: A-azepano-28-hydroxy derivatives (A), A-azepano-28-cinnamoyloxybetulin (B), A-seco-amino derivatives (C).
Figure 1.
Chemical structures of various active lupane-type derivatives: A-azepano-28-hydroxy derivatives (A), A-azepano-28-cinnamoyloxybetulin (B), A-seco-amino derivatives (C).
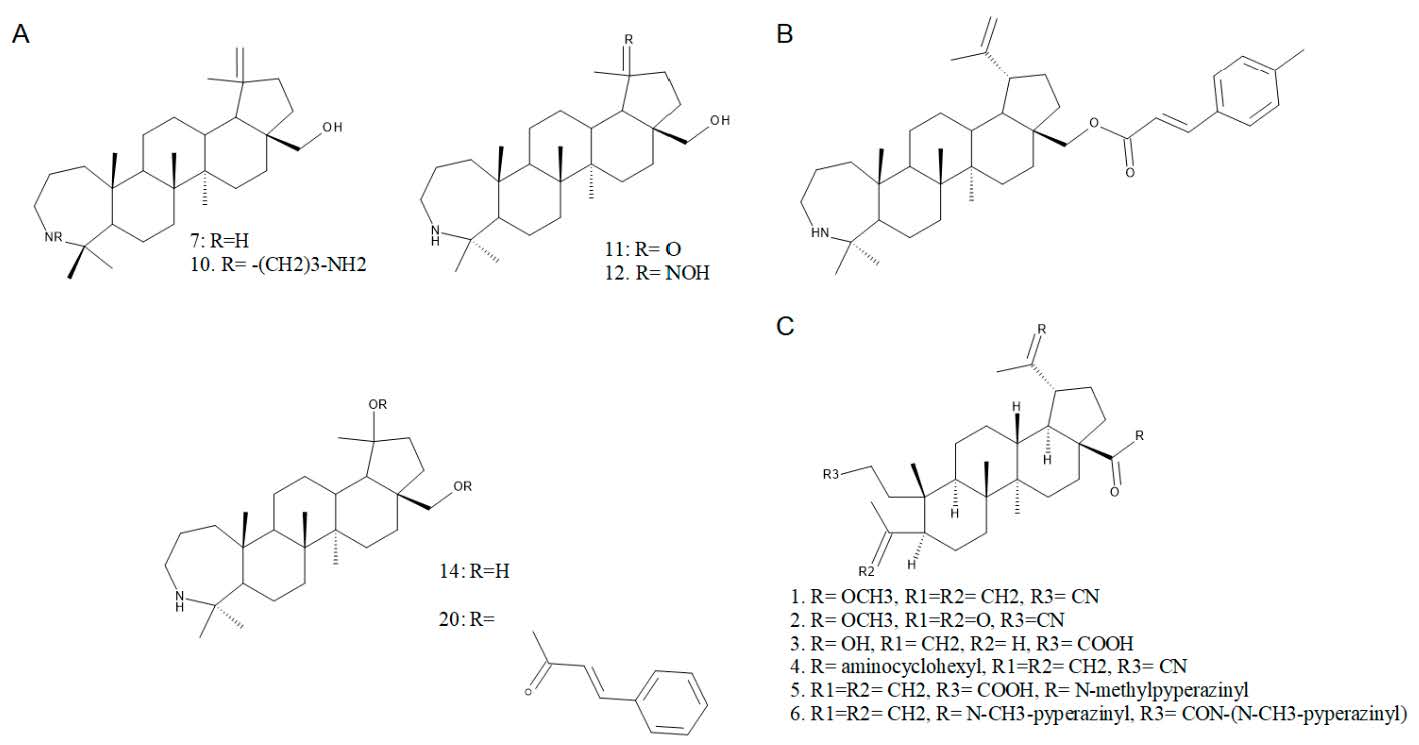
Figure 2.
Chemical structures of A-azepano lupine triterpene derivatives and lippiolides.
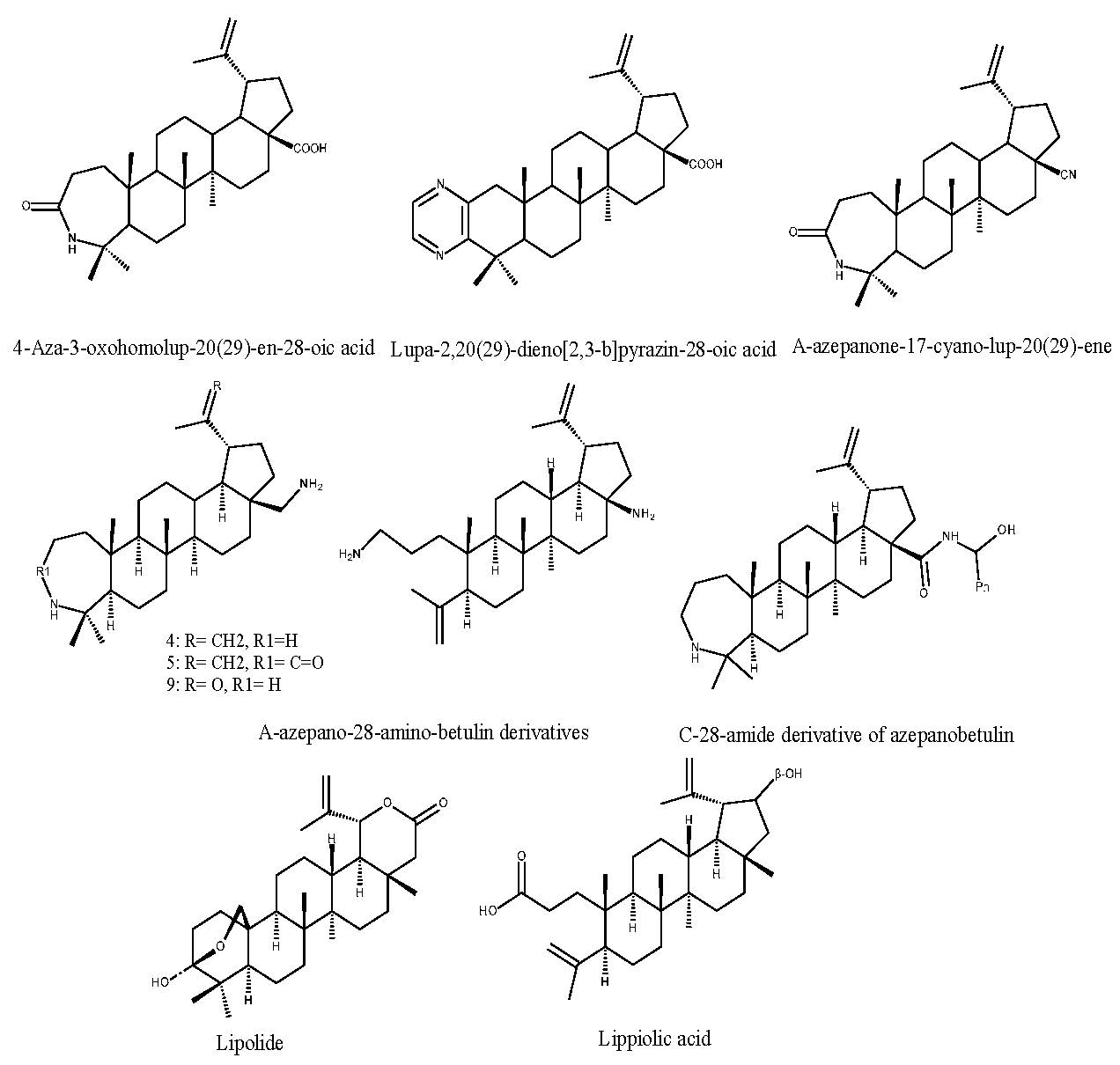
Figure 3.
Chemical structures of oleanane type triterpene derivatives.
© 2021 Copyright by the authors. Licensed as an open access article using a CC BY 4.0 license.
